PERFORMANCE DATA
Test results
Certain viruses and bacteria
iWave and NuShield products are designed to work with air handling systems to deliver the benefits of ionization. These tests measure the reduction of certain viruses and bacteria through a combination of in-air testing and surface testing. Measurements of the specimen are taken at regular intervals and compared to a control without the introduction of ionization. All tests were run using proprietary NPBI technology and conducted in third party laboratories.
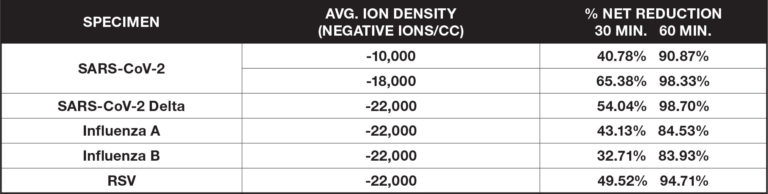
Airborne Organisms
These ionization tests measure the reduction of certain airborne viruses and bacteria by aerosolizing a test specimen into a large biosafety test chamber (BSL2 or BSL3) and suspending it in the air using mixing fans. Measurements of the specimen are taken at regular intervals and are compared to a control without the introduction of ionization.
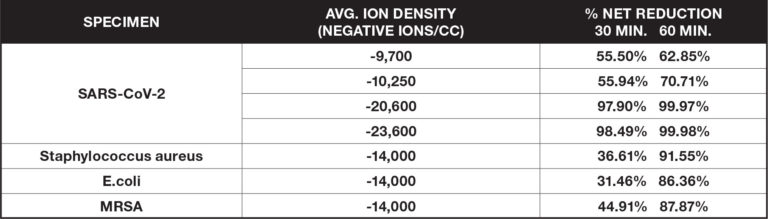
Surface Organisms
These ionization tests measure the reduction of certain airborne viruses and bacteria on surfaces by applying a specimen to glass slides, petri dishes or coupons and placing them within a large biosafety test chamber (BSL2 or BSL3). Measurements of the specimen are taken at regular intervals and are compared to a control without the introduction of ionization.
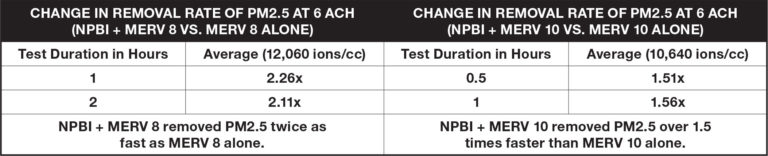
Airborne Particles
Test results demonstrate the additional reduction of particles in the air when NPBI is combined with mechanical filtration versus filtration alone. Particles from calibrated cigarettes were infused into a 10ft. x 10ft. x 10ft. chamber to simulate wildfire smoke. Testing occurred at six air changes per hour (ACH), consistent with ASHRAE guidelines
UL Certification
All NuShield models meet UL 2998 zero ozone emissions certification. All iWave models are UL 867 standard certified to have safe levels of ozone.
Disclaimer
Locations will vary, and clients should evaluate their individual application and environmental conditions when making an assessment regarding the technology’s potential benefits. iWave and NuShield products are not marketed as, nor cleared, by the FDA as medical devices.


